Crise pétrolière des années 1970, pollutions, émissions de gaz à effet de serre et menaces climatiques sont fréquemment englobées sous le terme de crise de l’énergie. Est-ce bien le cas ? Ne s’agit-il pas plutôt d’une crise de l’entropie ? Le physicien répond en expliquant un terme peu connu des non-spécialistes.
Everybody feels comfortable with the notion of energy, but entropy is often considered as a more difficult concept to understand. Energy is part of the general curriculum in elementary schools and high schools for all students, but only college students graduating in hardcore sciences will learn about entropy. This lack of education in a fundamental scientific concept, under the false pretense that it is too difficult for non-specialized students, presents us today with a very serious difficulty when we try to explain the exact nature of one of the major problems humanity faces today. We will argue that it is in fact easy to understand that this problem is really an entropy crisis rather than an energy crisis, and we will try to draw some of the consequences of this necessary shift in emphasis (Read: Une brève histoire de l’énergie and Qu’est-ce que l’énergie ?).
From a pedagogical point of view, the notions of energy and entropy should in fact be introduced at the same time in the curriculum, rather than separately. The origin of an earlier introduction of the notion of energy is mostly historical, while a simultaneous introduction is really necessary when we discuss the impact of our activities on the biosphere (Image 1).
It is common knowledge that in order to sustain life at the individual and society levels we need an energy input. This input is needed to compensate for the unavoidable increase of entropy, or disorder, in closed systems. Order in our bodies and order in society are maintained thanks to an energy input, and entropy is released to the outside world. Individuals need food energy, heat and so on. In addition, as we shall see later, complex societies consume energy well beyond the fundamental needs of the individuals.
![Image 2: Ludwig Boltzman - Source : Uni Frankfurt [Public domain], via Wikimedia Commons Image 2: Ludwig Boltzman - Source : Uni Frankfurt [Public domain], via Wikimedia Commons](https://www.encyclopedie-energie.org/wp-content/uploads/2015/10/art025_fig2_ludwig_boltzman.jpg)
Entropy released at all levels is not just of academic interest for experts in thermodynamics. It takes very practical and sometimes life threatening forms. Air pollution is a very common one. It can eventually reach dangerous levels over large areas and long periods of time. The way pollution proceeds is an illustration of a law established by Boltzmann, stating that entropy increases (logarithmically) as the number of possible locations molecules can occupy. When molecules emitted by a car exhaust enter into the atmosphere, a huge number of possible locations becomes available to them. Through a succession of collisions with the molecules that compose the atmosphere, they will eventually explore all these locations, because the accompanying entropy increase lowers a form of energy (free energy) of the biosphere. Carbon di-oxide molecules produced by the combustion of coal, liquid fuels and natural gas anywhere will eventually spread evenly in the planet’s atmosphere. Atmospheric pollution is a direct result of the entropy increase predicted by Boltzmann (Image 2).
1. Comparing different power plants from an entropy standpoint

Entropy release is a useful tool to compare different ways to produce energy. The entropy increase due to the combustion of conventional fuels is much larger than that resulting from that of nuclear fuels, and is therefore much more dangerous. In a study just released by the World Health Organization, it has been estimated that, in 2012, 7 million people died prematurely worldwide due to air pollution. This number is by orders of magnitude larger than the number of deaths due to the production of nuclear electricity over a much longer period. It is even much more than the number of deaths at Hiroshima and Nagasaki. This striking difference is a direct result of the law of Boltzmann. While the spread of carbon di-oxide and other combustion gases in the atmosphere cannot be stopped, spent nuclear fuel can be contained because it is in solid or liquid form. Therefore the entropy released by nuclear power plants is negligible. Leaks are possible, but they are the exception, while in the case of conventional gases they are the absolute rule. Burning coal, oil or natural gas to produce electricity is infinitely more damaging to our health than producing nuclear electricity (Image 3).
Renewable energy sources are not immune to entropy release. To provide a constant power supply in spite of their intermittency, they need to be backed up by conventional power plants if one has decided to ban nuclear electricity. Closing down nuclear power plants and replacing them by wind or solar power plants backed up by conventional power plants will in the end result in an increased entropy release, and more damage to our health.
One may wonder why it has taken so long for the air pollution problem to attract wide attention. Maybe this is because, as all forms of entropy release, pollution is a non-local and time dependent phenomenon, whose effects are often not immediately visible on a large scale. A contrario, consumption of energy is local and immediate. When we burn petrol in our car, we immediately know that it is gone, this is a local and instantaneous event. If we want to keep driving, we must pay to fill up again the gas tank once it is empty. Gas price is an immediate worry. But the effect of pollution is not felt, and not paid for, immediately. Carbon and nitrogen oxide molecules as well the particulates that our car leaves behind will not bother us as we drive along. But once released they will spread progressively in the atmosphere. Nothing will stop them. As many cars participate in the traffic, the particulates that will get eventually inside our lungs are probably not those that we have emitted. So who is to blame? And who should pay for the damage to our health, and that of others? And what about the effect on the climate of the released green house gases such as carbon dioxide? How many future generations will suffer the consequences of climate disorder? And what about pollution of rivers and underground water reservoirs, unavoidable due to the same law of Botzmann? These are the kind of hard questions raised by the increase of entropy in the biosphere.
2. The so-called energy crisis
The term “Energy crisis” was I believe born from the oil embargo that followed the Yom Kippur war. At that time, oil was the fuel of the future. It was cheaper than coal and its reserves were abundant. Power plants had turned from coal to oil. Oil had also replaced coal for heating, being both cheaper and cleaner. Nuclear energy had some success but could not possibly compete with cheap oil. More cars on the roads needed increasing quantities of gas to keep moving. A sudden oil embargo was for western countries a terrifying prospect.
As it turned out, this was not really a fundamental energy crisis. It was just a temporary oil crisis. While in 1975 much more carbon dioxide was released from burning oil than from burning coal, today the situation is reversed. While oil consumption increases only slowly, coal consumption increases quickly. It is cheap and abundant. World coal reserves are sufficient for several centuries. The exploitation of natural gas, of shale gas and shale oil are on the rise. Coal, natural gas, shale gas and shale oil are much more broadly shared around the five continents than is oil. For many years to come, there is no risk of being short of energy supplies, even without counting renewables and nuclear energy. There never was an energy crisis, and there will not be one in the foreseeable future.
As I have shown in detail elsewhere the real threat that we face is an entropy crisis[1]. Its effects are already quite visible. But before we come back to the present situation, let us have a quick look at the history of the biosphere in terms of energy and entropy.
3. Entropy evolution in the biosphere
![Image 4: Carboniferous age - Source: J. F. Horrabin (Illustrator) [Public domain], via wikimedia Commons Image 4: Carboniferous age - Source: J. F. Horrabin (Illustrator) [Public domain], via wikimedia Commons](https://www.encyclopedie-energie.org/wp-content/uploads/2015/10/art025_fig4_carboniferous_age.jpg)
Five hundred million years ago the concentration of carbon di-oxide in the atmosphere was about 25 times larger than it is today, temperatures were about 10 degrees Celsius higher and there was no vegetation on earth. The apparition of an ozone layer then allowed the propagation of vegetation on dry land through photosynthesis, which lead progressively to the reduction of the carbon di-oxide content in the atmosphere and to lower temperatures due to a weaker green house effect. Part of the wood produced through photosynthesis transformed into coal deposits, particularly during the carboniferous age that lasted from 350 million years to 250 million years ago (Image 4). Oil and gas deposits were also formed through the accumulation of organic matter, maybe marine organisms. Overall, entropy in the biosphere reduced and life expanded under the energy input of solar radiation. For this long period of time entropy released by living organisms and other transformations was more than compensated by the solar energy input.
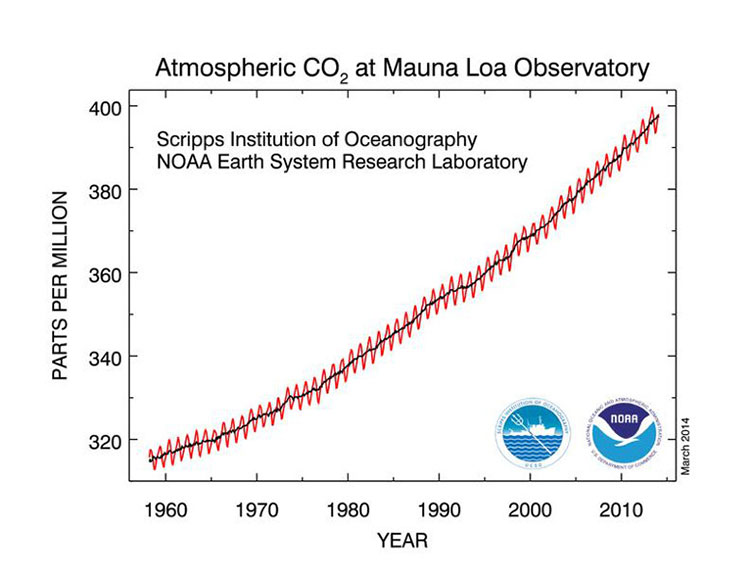
The current massive use of fossil fuels is now reversing this trend. More entropy is released into the biosphere than can be compensated by photosynthesis. A measure of this reversal is given by the increase of carbon di-oxide concentration in the atmosphere, which has been monitored very precisely since 1958 at the Mauna Loa observatory in Hawai. Detailed records of this concentration for the last 500, 000 years are available show oscillations between 180 ppm and 280 ppm. It was up to 315 ppm in 1958, slightly above the range of these oscillations. Today it is close to 400 ppm, far outside this range. There is no doubt that this increase does not fit with previously known variations, therefore its origin must be found in the anthropogenic massive combustion of fossil fuels. At the current rate of increase the carbon di-oxide concentration will reach about 600 ppm in 2100, or about a factor of 2.5 higher than the median concentration in the last several million years. This will take us back to where the biosphere was about 100 million years ago, which gives us an idea of the impact of massive fossil fuel burning: it is significant on a geological time frame.
The increase of carbon di-oxide concentration is a measure of our global entropy release. It is taking us into unchartered territory. Humanity has enjoyed during the last 10,000 years a period of exceptional climate stability, which has allowed civilization to flourish. No one can predict today the possible consequences of such a strong and rapid increase of the carbon di-oxide content in the atmosphere. Global warming is only one of them, and may not be the most important one. Climate instability may be a more serious one. It is therefore prudent and some will say imperative to reduce our entropy release, in order to get back to conditions under which it is compensated by the incoming solar radiation. This would require reducing the global level of carbon emissions down to where they were about 50 years ago, when the concentration of carbon di-oxide was still close to the upper range of its normal fluctuations, 280 ppm (Image 5).
Entropy release is related to the amount of energy we consume, and to the efficiency of the transformations involved. Both aspects should be examined.
4. How much energy do we need?
Excluding food, the amount of power spent per capita in Nigeria is only 43 W. In developed societies it is typically one hundred times larger. Where does this huge difference come from?
Table 1 itemizes the daily energy needs of a household of 4 in a typical Euro country. Excluding food, it is about 100 kWh, which corresponds to a power of about 1kW per capita, 20 times larger than in Nigeria. This difference is easily understood: most people in Nigeria do not have electrical appliances, do not use sanitary hot water, the weather does not require home heating, air conditioning is not in general use and people do not use private cars for transportation. Note however that the power spent in the form of food consumption is the same for everybody, about 100 W.
Table 1: Daily Energy needs for a family of 4 in kWh
| Food energy
Based on an average power consumption of 150W, taking into account a modicum of physical activity |
10 |
| Electrical appliances
Includes refrigerator, washing machines, television, personal computer, light etc. |
10 |
| Hot water
For sanitary use |
10 |
| Home heating
For a well insulated single family house of 160 sq. meters where 1000 liters of fuel or equivalent are consumed per year |
30 |
| Transportation
Corresponding to an annual driving of 15,000 km driven in a car consuming on the average 10 liters of petrol for 100 km. There are 10 kWh of stored chemical energy in one liter of petrol. |
45 |
| Total | 110 |
There is however a major surprise when we compare the power consumption at the household level to the overall consumption at the society level: in a euro-country the power used per capita at the household is five times smaller than the power used per capita at the society level. This factor of five is the problem. It is crucially important to understand why so much more power is being spent at the society level than at the private level. Humanity could afford spending 1 kW per capita, this was about the power spent 50 years ago before the amount of carbon di-oxide started to deviate from its historical pattern. But it cannot afford spending five times more. Not because the required energy is not available, but because the world would choke under the effect of entropy release for lack of breathing quality air and drinkable water (Read: Les besoins d’énergie).
The general idea that our world has limited natural resources and therefore cannot sustain a larger population has been around for a long time. It is however a wrong idea. Resources are available for an even larger population, at least for many centuries to come (Read: Charbon minéral : géologie, ressources et réserveset Géologie et gé0dynamique des hydrocarbures). The limiting factor is that the natural low entropy environment (“clean environment”) from which we have benefited up to recently is quickly vanishing. What will limit population increase is not the lack of energy, but the increase in entropy in the biosphere. Coal and other resources are not the problem. The problem is in the end the limited volume of the biosphere.
Reducing the power spent at the level of the household is certainly possible: more energy efficient appliances are becoming available, homes are being better insulated, car mileage is improving (Read: Les politiques d’efficacité énergétique). This is all very good, but quite insufficient. The major challenge is to reduce the 4 kW spent per capita at the society level in developed countries. As they are today, developed societies are not a viable development model for the rest of humanity.
5. Re-organizing society
Society must be re-organized to reduce the “overhead entropy” that is released at its level, compared to the entropy released at the private individual level. It is really absurd to tell people that they must reduce the amount of energy they spend, or in our language the amount of entropy they release – while at the same time ignoring the fact that most of the entropy is being released beyond their control.
It is a major challenge to bring under control the entropy released at the society level. To start with, one should understand in some detail how this occurs.
For example, the urban society model should be analyzed in terms of the entropy it releases, as compared to other more dispersed societies. On the one hand people, food and energy must be transported over long distances towards urban centers. The resulting air pollution affects a large number of people. On the other hand, there are economies of scale. Buildings can be more easily and effectively thermally insulated than individual homes. Urban mass transportation releases less entropy than individual transportation. Entropy release in mega-cities comprising large suburban areas should also be considered. They may combine disadvantages of urban and rural societies, and few of their advantages.
The role of government should also be analyzed. How much entropy release does it contribute through the operation of its administration, government owned buildings, transportation of public employees, maintaining police and defense forces, public services such as schools and hospitals, keeping roads in a good state of repair?
These are only some of the questions one should consider when trying to track down the reasons why entropy release at the society level is so much larger than that released at the individual level.
6. Technology can be of help
Obviously improved technology can lower the amount of entropy released per unit of energy spent. Efforts in this direction are under way, there is no point in discussing them here in detail as this is common knowledge. One simple point should however be stressed. A technology that allows a lower level of entropy release will in general be more expensive than a less effective one.
Electricity production is a good example. The cheapest way to produce electricity today is to burn coal. It also gives the highest amount of entropy release per kWh produced.
Transportation is another good example. Diesel cars are cheaper to buy than hybrids.
Poorly insulated buildings are cheaper to build than better insulated ones.
Wood burning is the cheapest way to cook and to heat homes. It also gives the highest air pollution, inside and outside homes.
These examples make the same point: at the present time there is no price tag attached to entropy release. It is free of charge. We only pay for energy consumed, not for entropy released (Read: Marchés de l’énergie : prix et régulation).
It is therefore clear that developing countries, which cannot afford the better technologies that reduce entropy release because of their cost, will keep being the most polluted (and polluting) ones.
Even amongst developed countries, there can be differences. Richer ones will be able to invest more in low entropy releasing technologies. This can be seen inside Europe, with corresponding differences in the levels of air pollution. Berlin is less polluted than Paris, itself less polluted than Rome and Athens (Read: La transition énergétique : un enjeu majeur pour la planète).
Notes and References
[1] See The entropy crisis. World Scientific, 2008.
L’Encyclopédie de l’Énergie est publiée par l’Association des Encyclopédies de l’Environnement et de l’Énergie (www.a3e.fr), contractuellement liée à l’université Grenoble Alpes et à Grenoble INP, et parrainée par l’Académie des sciences.
Pour citer cet article, merci de mentionner le nom de l’auteur, le titre de l’article et son URL sur le site de l’Encyclopédie de l’Energie.
Les articles de l’Encyclopédie de l’Énergie sont mis à disposition selon les termes de la licence Creative Commons Attribution – Pas d’Utilisation Commerciale – Pas de Modification 4.0 International.